Rocatinlimab Effective for Moderate-to-Severe Atopic Dermatitis
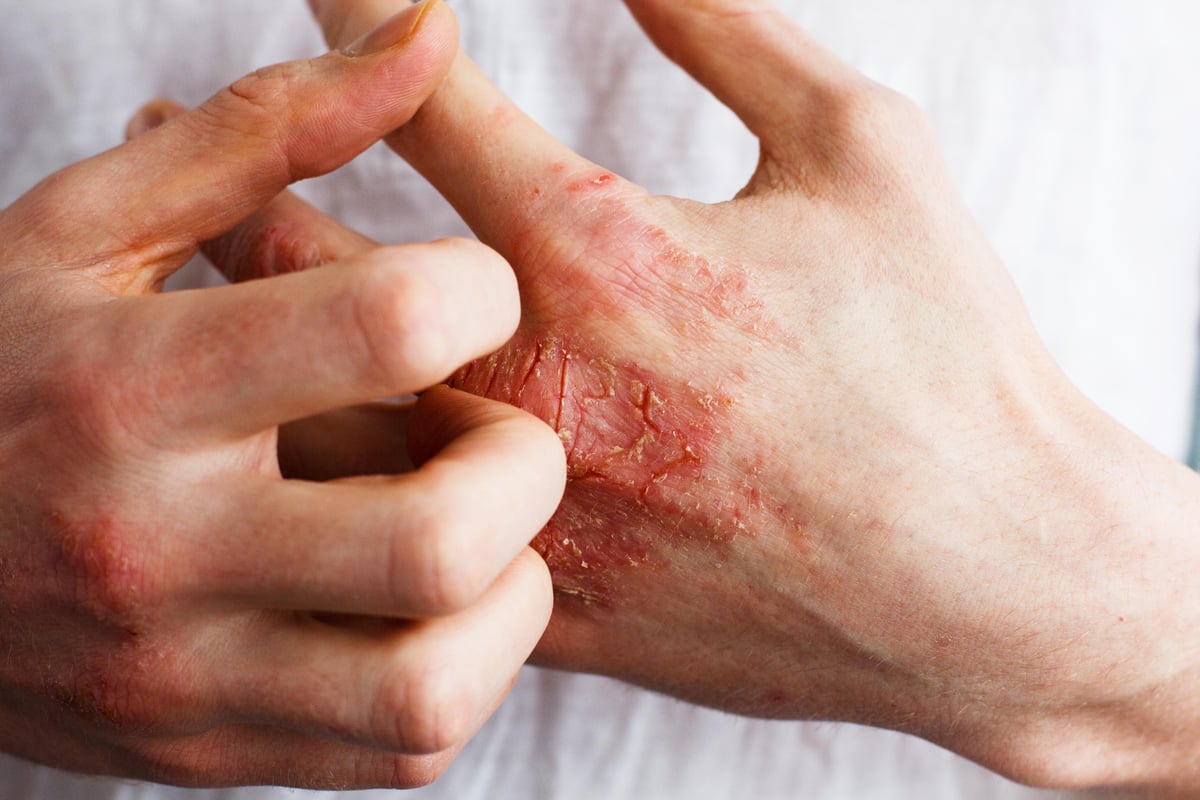
THURSDAY, Dec. 15, 2022 (HealthDay News) -- The anti-OX40 antibody rocatinlimab is effective for adults with confirmed atopic dermatitis with moderate-to-severe disease activity, according to a study published online Dec. 9 in The Lancet.
Emma Guttman-Yassky, M.D., from the Icahn School of Medicine at Mount Sinai in New York City, and colleagues conducted a double-blind, placebo-controlled study at 65 secondary and tertiary sites in the United States, Canada, Japan, and Germany. Eligible adult patients with confirmed atopic dermatitis with moderate-to-severe disease activity were randomly assigned to receive subcutaneous rocatinlimab every four weeks (150 or 600 mg) or every two weeks (300 or 600 mg) or subcutaneous placebo up to week 18, followed by an 18-week active-treatment extension and 20-week follow-up. The primary end point at week 16 was percentage change from baseline in the Eczema Area and Severity Index (EASI) score.
A total of 274 patients were enrolled between Oct. 22, 2018, and Oct. 21, 2019, and were randomly assigned to one of the rocatinlimab groups (217 patients; 79 percent) or placebo (57 patients; 21 percent). The researchers observed significant least-squares mean percent reductions in the EASI score at week 16 in all rocatinlimab groups compared with placebo (−48.3, −49.7, −61.1, and −57.4 versus −15.0 for rocatinlimab 150 mg every four weeks, 600 mg every four weeks, 300 mg every two weeks, and 600 mg every two weeks, respectively, versus placebo).
"At week 36, all participants had been on the treatment for at least 18 weeks," Guttman-Yassky said in a statement. "By this time, we saw that while the drug achieved the primary end points in all doses versus the placebo, it's also a drug that improves over time, which is really unusual and unique among currently available treatment options."
Several authors disclosed financial ties to pharmaceutical companies, including Kyowa Kirin, which manufactures rocatinlimab and funded the study.
Abstract/Full Text (subscription or payment may be required)
Editorial (subscription or payment may be required)
Related Posts
Not Enough Time in a Day for Recommended Primary Care
TUESDAY, Aug. 9, 2022 (HealthDay News) -- Primary care providers (PCPs) do not...
Walk the Roads at Your Own Risk as Pedestrian Deaths Keep Climbing
THURSDAY, April 7, 2022 (HealthDay News) -- America's roads are getting ever...
¿Podrían los tiburones ayudar a luchar contra los coronavirus del futuro?
https://consumer.healthday.com/b-12-21-could-shark... Credit: HealthDay
Radiologists’ Group Pushes for Breast Cancer Risk ‘Assessment’ by Age 25
THURSDAY, May 4, 2023 (HealthDay News) -- While the typical recommendation is...
