Half of Patients With Newly Diagnosed Vitiligo Do Not Receive Treatment
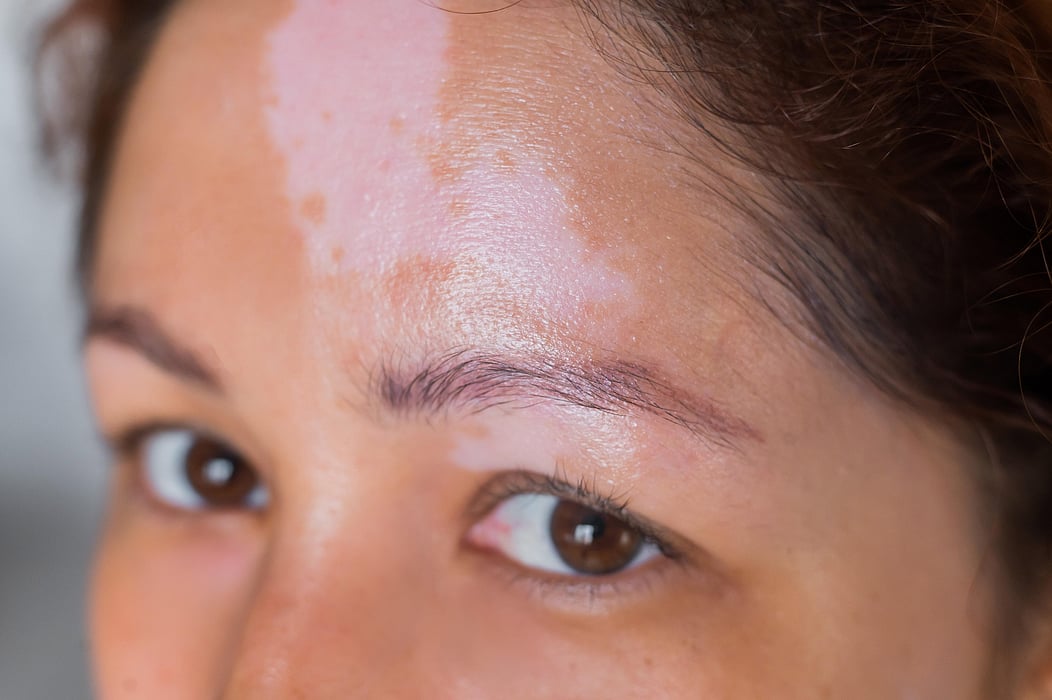
TUESDAY, Oct. 17, 2023 (HealthDay News) -- A high proportion of patients with newly diagnosed vitiligo do not receive any treatment, according to a study recently published in Dermatology and Therapy.
David Rosmarin, M.D., from the Indiana University School of Medicine in Indianapolis, and colleagues retrospectively analyzed claims data from the Merative MarketScan Research Databases. The analysis included data from 19,335 patients aged 12 years and older with newly diagnosed vitiligo.
The researchers found that 49.9 percent of patients did not receive any treatment in the 12-month follow-up. Among those initiating treatment, switching was minimal, with the most frequent first-line treatments being high-potency topical corticosteroids (25.4 percent), oral corticosteroids (23.1 percent), and topical calcineurin inhibitors (TCIs; 14.7percent). Among adolescents initiating treatment, TCI was the most frequently received first-line therapy (30.9 percent). During follow-up, patients with moderate-to-severe vitiligo were very likely to receive treatment, with only 1.5 percent not receiving treatment. Time to first medication claim ranged from 51.9 days for TCI to 178.6 days for systemic immunosuppressants. The mean total days supplied ranged from 14.4 days for oral corticosteroids to 121.0 for immunosuppressants.
"To help better understand the needs of patients with vitiligo, future studies need to examine which demographic and clinical characteristics are associated with not receiving treatments for vitiligo," the authors write.
Rosmarin disclosed ties to pharmaceutical companies, including AbbVie, which funded the study.
Related Posts
Male Teens With Sickle Cell Disease Unaware of Related Fertility Issues
FRIDAY, April 22, 2022 (HealthDay News) -- Many adolescent and young men with...
Exercise Might Boost Outcomes for People Battling Esophageal Cancer
THURSDAY, Feb. 3, 2022 (HealthDay News) -- Alan Holman didn't stop exercising...
Even Minor Traffic Accidents Can Raise a Woman’s Odds for Birth Complications
TUESDAY, March 21, 2023 (HealthDay News) -- “Baby on Board” warning stickers...
Monkeypox Infection Rare in Children, Adolescents Younger Than 18
MONDAY, Nov. 7, 2022 (HealthDay News) -- Monkeypox infection is rare among...
