FDA Warns Amazon, Other Vendors About Sale of Skin Tag Removal Products
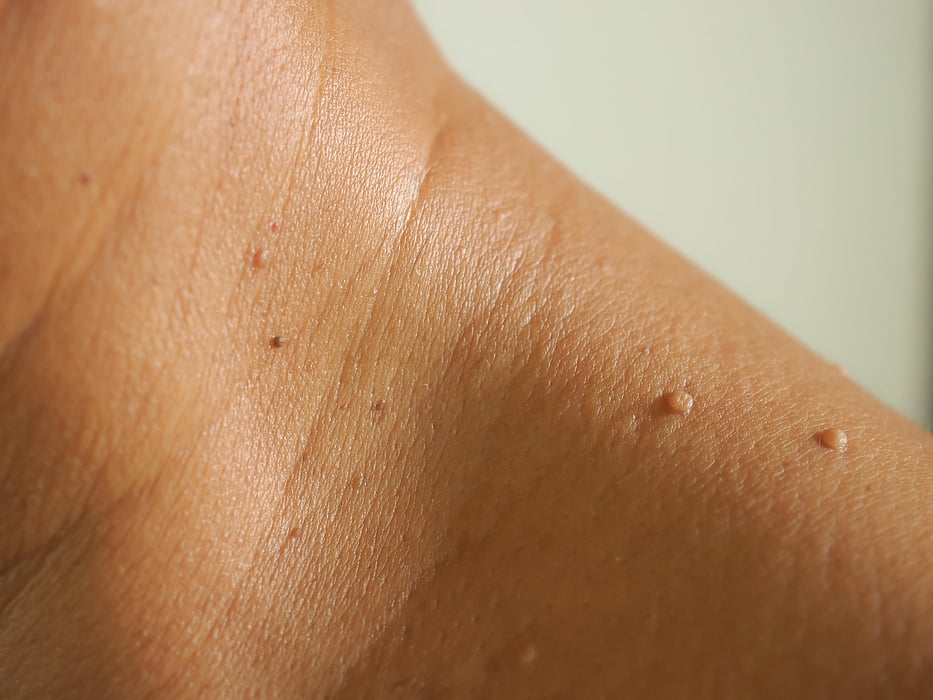
WEDNESDAY, Aug. 10, 2022 (HealthDay News) -- The U.S. Food and Drug Administration on Tuesday issued warning letters to three companies, including Amazon, for selling unapproved products for removing moles and skin tags.
No over-the-counter medications have FDA approval for that purpose, and the Food, Drug, and Cosmetic Act prohibits interstate sale of unapproved drugs and cosmetics.
"It is the FDA's duty to protect public health from harmful products not approved for the U.S. marketplace," Donald Ashley, director of the compliance office in the FDA Center for Drug Evaluation and Research, said in a statement. "This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law."
Besides Amazon, the FDA issued warning letters to Ariella Naturals and Justified Laboratories. The agency emphasized that it has not examined the mole and skin tag removal products sold by these companies for safety, effectiveness, or quality.
The warning letters put the companies on notice that failure to address the violations cited by the FDA may result in legal action, including seizure. Once they receive the warning letter, the companies have 15 days to inform the agency of any corrective measures they have taken.
The FDA said Tuesday that it will continue to use all the tools available to protect public health and remove fraudulent or unproven drug products from the American market. The warning letters were just the first step.
Related Posts
Menthol Ups Smoking Frequency in Youth
FRIDAY, June 10, 2022 (HealthDay News) -- The addition of menthol to cigarettes...
El respaldo social después de una estadía en la UCI es vital para la supervivencia de las personas mayores
MIÉRCOLES, 15 de septiembre de 2021 (HealthDay News) -- Los adultos mayores que...
Una mujer podría haberse librado del VIH de forma natural… ¿pero cómo?
MARTES, 16 de noviembre de 2021 (HealthDay News) -- Los investigadores han...
Acetazolamide Helps Treat Volume Overload in Heart Failure
MONDAY, Aug. 29, 2022 (HealthDay News) -- For patients with acute decompensated...
