FDA Warns Against Using Bogus Treatments for Molluscum
MONDAY, June 5, 2023 (HealthDay News) -- Patients may be tempted to treat little skin bumps on their own, but that can delay proper diagnosis and treatment that may work better, federal regulators caution.
Products marketed as treatments for molluscum have not been approved by the U.S. Food and Drug Administration, the agency warned. There are no approved treatments in either prescription or over-the-counter form for the condition, which will typically go away on its own in six to 12 months but could last up to five years.
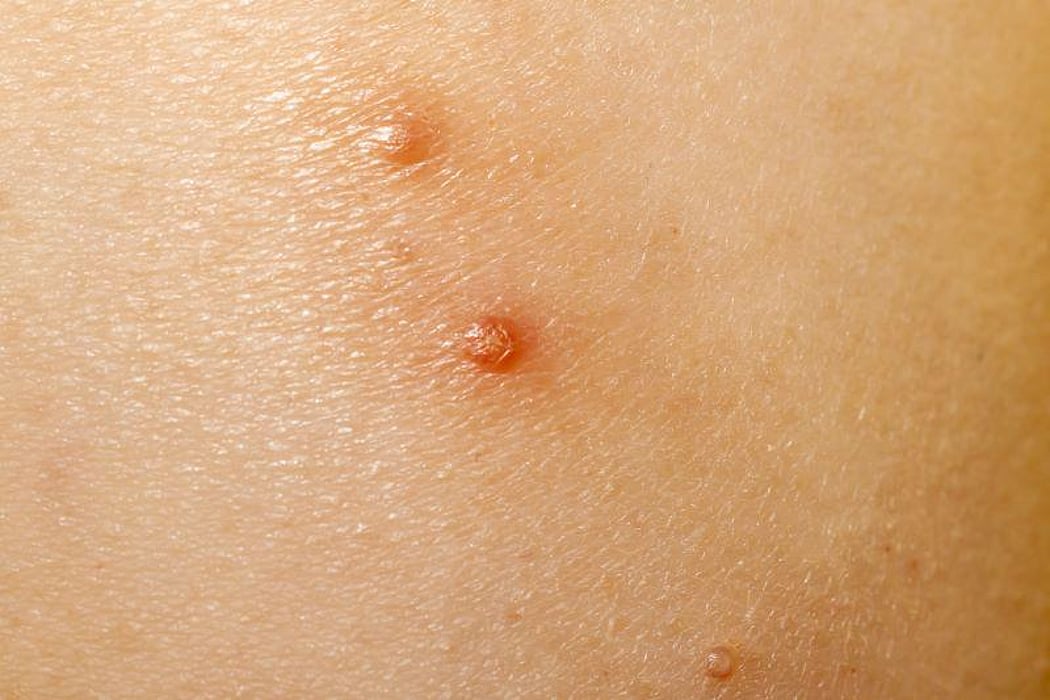
Molluscum can grow alone or in groups almost anywhere on the skin, including the face, neck, arms, legs, abdomen, and genital area. They are rarely on the palms of the hands or soles of the feet. Sometimes these bumps itch and get irritated. Patients with a weakened immune system may have larger or more bumps. They are more common in children, but can happen in teens and adults.
Molluscum is spread by skin-to-skin contact, including sexual contact, and by sharing clothes or infected objects such as sports equipment. Staying clean, including hand washing, is the best way to prevent them.
Unapproved products are unlikely to actually have the impact they claim and may cause bad reactions, such as skin reddening, abrasion from skin scratching, and permanent scarring. It is also possible to have an allergic reaction to products, such as those that contain essential oils.
Related Posts
Health Highlights: March 9, 2023
Two healthy diets may help prevent dementia. Seniors who followed either the...
Many Localities Not Ready for Launch of 988 Mental Health Hotline
THURSDAY, June 9, 2022 (HealthDay News) -- Half of jurisdictions may not be...
Buyer Beware: Bogus Flu Meds Are Out There
MONDAY, Dec. 26, 2022 (HealthDay News) -- With flu rampant in the United States,...
Too Many Americans Are Getting ‘Low-Value’ Medical Tests, Procedures
WEDNESDAY, Feb. 23, 2022 (HealthDay News) -- When your cardiologist orders a...
