FDA Wants More Data on First Needle-Free Antidote for Severe Allergic Reactions
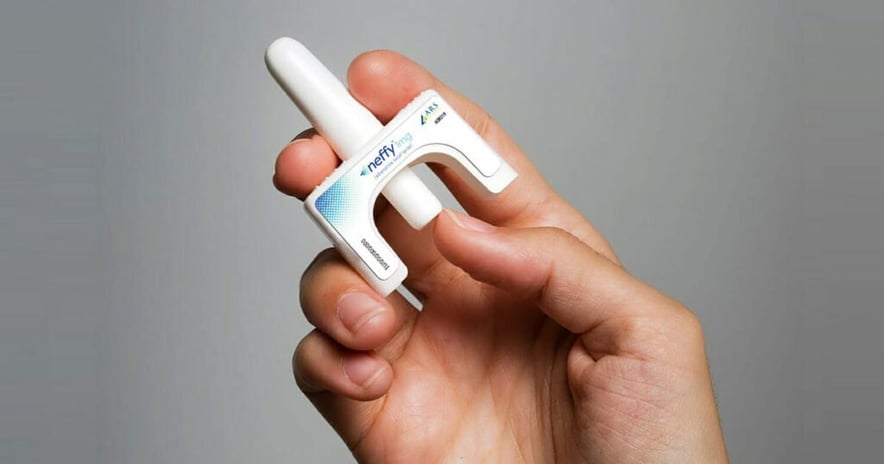
WEDNESDAY, Sept. 20, 2023 (HealthDay News) -- In a surprising move, the U.S. Food and Drug Administration has opted not to approve a needle-free alternative to the EpiPen for emergency treatment of severe allergic reactions.
Approval of the Neffy (epinephrine) nasal spray was widely anticipated. An FDA advisory panel voted to recommend approval of the drug for children and adults in May. While the FDA is not obligated to follow the advice of its advisory panels, it usually does. Instead, the FDA told the drug's maker, ARS Pharmaceuticals, that it needed to conduct another study on the drug before it is approved, the company said in a statement late Tuesday night.
"We are deeply disappointed that this action further delays the availability of Neffy for the millions of people who are at risk of a potentially life-threatening severe allergic reaction," said Richard Lowenthal, cofounder, president, and CEO of ARS Pharma.
"We stand by the totality of the Neffy data package in a comprehensive registration program that was aligned upon with FDA and believe strongly in the value Neffy can provide for patients, families, and caregivers living daily with severe allergic reactions," he said in a company statement, adding that his firm will aim to complete the requested trial as soon as possible.
The news was unwelcome on the front lines of health care. "It's certainly disappointing as we were hoping to have another option for people at risk of severe allergic reactions," Scott Sicherer, M.D., director of the Elliot and Roslyn Jaffe Food Allergy Institute at Mount Sinai in New York City, told HealthDay.
"Neffy would have been the first needle-free option for patients to treat severe allergic reactions, but we still have the injectable epinephrine devices available," Payel Gupta, M.D., an assistant clinical professor at SUNY Downstate Medical Center in New York City and volunteer medical spokeswoman for the American Lung Association, told HealthDay. "A needle-free option would be great for people with needle phobia or who are anxious about injecting themselves."
Related Posts
Bidirectional Association for Ménière Disease, Osteoporosis
THURSDAY, Dec. 22, 2022 (HealthDay News) -- Adults with Ménière disease (MD)...
FDA: No Useful Monoclonal Antibody Treatments Left Against New COVID-19 Variants
MONDAY, Dec. 5, 2022 (HealthDay News) -- The last of six COVID-19 monoclonal...
AHA News: Donar sangre beneficia tanto al receptor como al donante, y ahora es un momento crítico
MIÉRCOLES, 23 de febrero de 2022 (American Heart Association News) -- Cada...
‘Walking Miracles’: Born With Lungs Reversed, They Suffered Until Getting Double-Organ Transplants
TUESDAY, Aug. 8, 2023 (HealthDay News) -- Dennis Deer woke from surgery in utter...
