FDA Approves Dupixent for Prurigo Nodularis
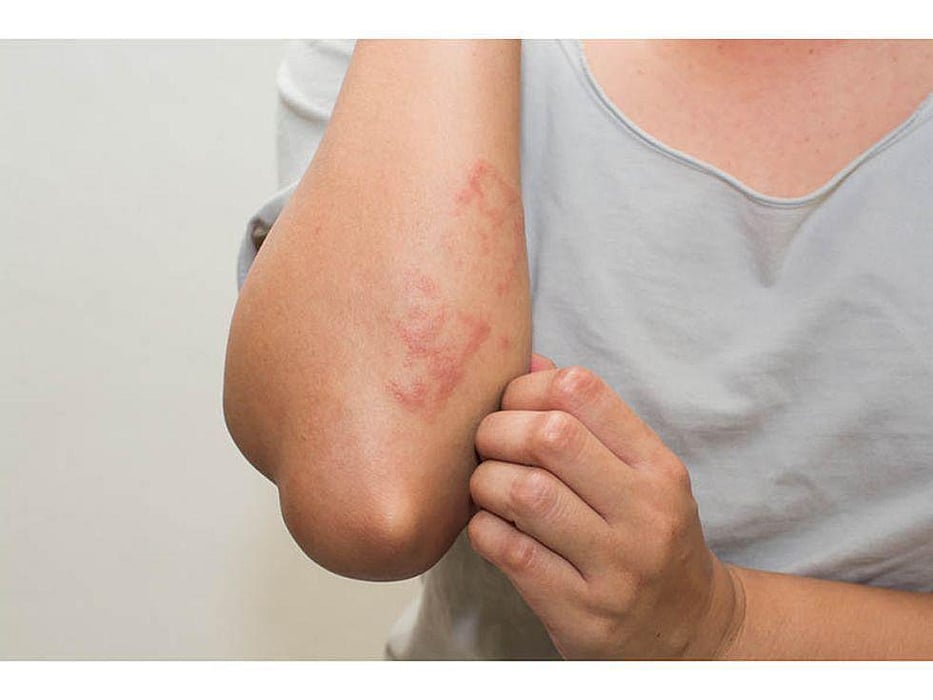
FRIDAY, Sept. 30, 2022 (HealthDay News) -- The U.S. Food and Drug Administration approved Dupixent (dupilumab) as the first treatment for adults with prurigo nodularis, according to Regeneron Pharmaceuticals.
Prurigo nodularis, a chronic, debilitating skin disease with underlying type 2 inflammation, is estimated to impact 75,000 U.S. adults and, until now, had no systemic treatment options.
The approval was based on priority review using data from two direct-to-phase 3 trials evaluating the efficacy and safety of Dupixent. In the two trials, more patients randomly assigned to Dupixent experienced a clinically meaningful reduction in itch from baseline to both 12 weeks (44 and 37 percent, respectively, versus 16 and 22 percent with placebo) and 24 weeks (60 and 58 percent, respectively, versus 18 and 20 percent for placebo). Compared with placebo (18 and 16 percent), more than twice as many Dupixent patients (48 and 45 percent) achieved clear or almost clear skin at 24 weeks. The safety profile was consistent with use of Dupixent for other dermatological conditions and included nasopharyngitis, conjunctivitis, herpes infection, dizziness, muscle pain, and diarrhea.
"Patients living with prurigo nodularis must often contend with dozens, if not hundreds, of itchy and painful nodules covering their body," George D. Yancopoulos, M.D., Ph.D., president and chief scientific officer at Regeneron, said in a statement. "With this approval, those suffering with prurigo nodularis finally have a medicine to address the debilitating signs and symptoms of the disease."
Related Posts
Review Compares Diagnostic Strategies for Assessment of Coronary Artery Disease
TUESDAY, June 6, 2023 (HealthDay News) -- Coronary computed tomography...
Not Just Brushing: 10 Ways to Start Caring for Baby Teeth
SATURDAY, Feb. 4, 2023 (HealthDay News) -- Even the tiniest teeth can decay,...
Telehealth for Opioid Use Disorder Helped Curb Fatal ODs During Pandemic
FRIDAY, March 31, 2023 (HealthDay News) -- Telehealth appointments — meetings...
2011 to 2020 Saw Steady Improvement in Childhood Vaccine Adherence
MONDAY, May 22, 2023 (HealthDay News) -- Adherence to national guidelines for...
