CDC Gives Full Approval to RSV Vaccine for People Over 60
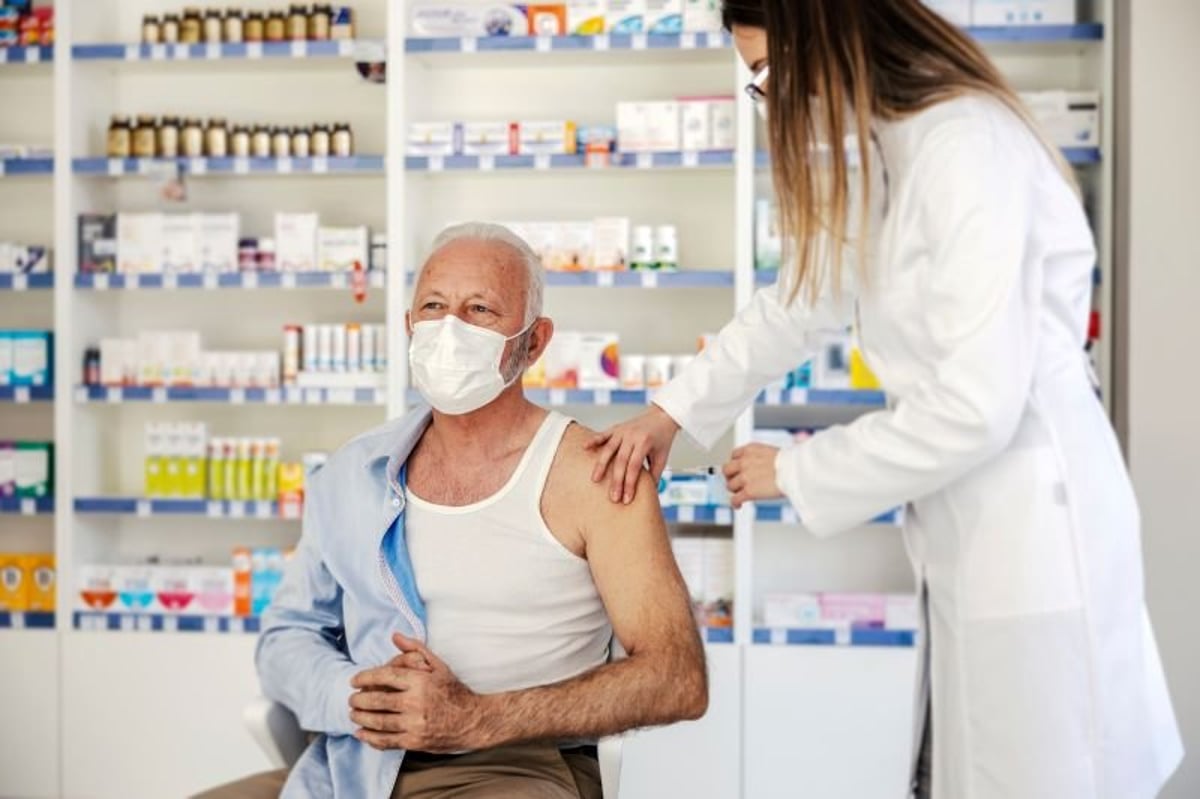
THURSDAY, June 29, 2023 (HealthDay News) -- Americans ages 60 and up can get their vaccine for respiratory syncytial virus (RSV) this fall, the U.S. Centers for Disease Control and Prevention has announced.
On Thursday, Rochelle Walensky, M.D., the outgoing CDC director, gave her signature to a recommendation made last week by an advisory panel of outside experts for a single dose of the vaccines made by Pfizer and GlaxoSmithKline. The U.S. Food and Drug Administration sanctioned the shots last month for adults 60 and older. The CDC added in a statement that it is advising seniors to first consult with their doctors to determine if the vaccine is right for them.
While some, including Robert Blancato, executive director of the National Association of Nutrition and Aging Services Programs, wanted the CDC to give a stronger vaccine recommendation for those ages 65 and up, the advisory panel offered the weaker endorsement after raising questions, the Associated Press reported. Those included how well the vaccine worked in those who were most frail, whether there might be a need for boosters and costs.
Pfizer has not revealed their pricing plan. GSK plans to charge between $200 and $295 a shot.
RSV can appear like the common cold, but can be dangerous for infants, children and the elderly, the AP reported. Those adults considered most at risk have chronic heart or lung disease, a weakened immune system, or live in a long-term care facility.
Related Posts
Fasting Diet Could Help Folks With Type 2 Diabetes
TUESDAY, July 26, 2022 (HealthDay News) -- Intermittent fasting might help...
Fish on Your Plate May Keep Your Brain Sharp
THURSDAY, Nov. 4, 2021 (HealthDay News) -- Older folks who eat fish a couple of...
Ser vegetariano podría depender de tus genes
JUEVES, 5 de octubre de 2023 (HealthDay News) -- Ser vegetariano es una...
Pet Dogs Can Alert Owners to Epileptic Seizures
WEDNESDAY, Sept. 8, 2021 (HealthDay News) -- Sit. Fetch. Stay.Detect...
