IUD Placement at Two to Four Weeks Postpartum Noninferior
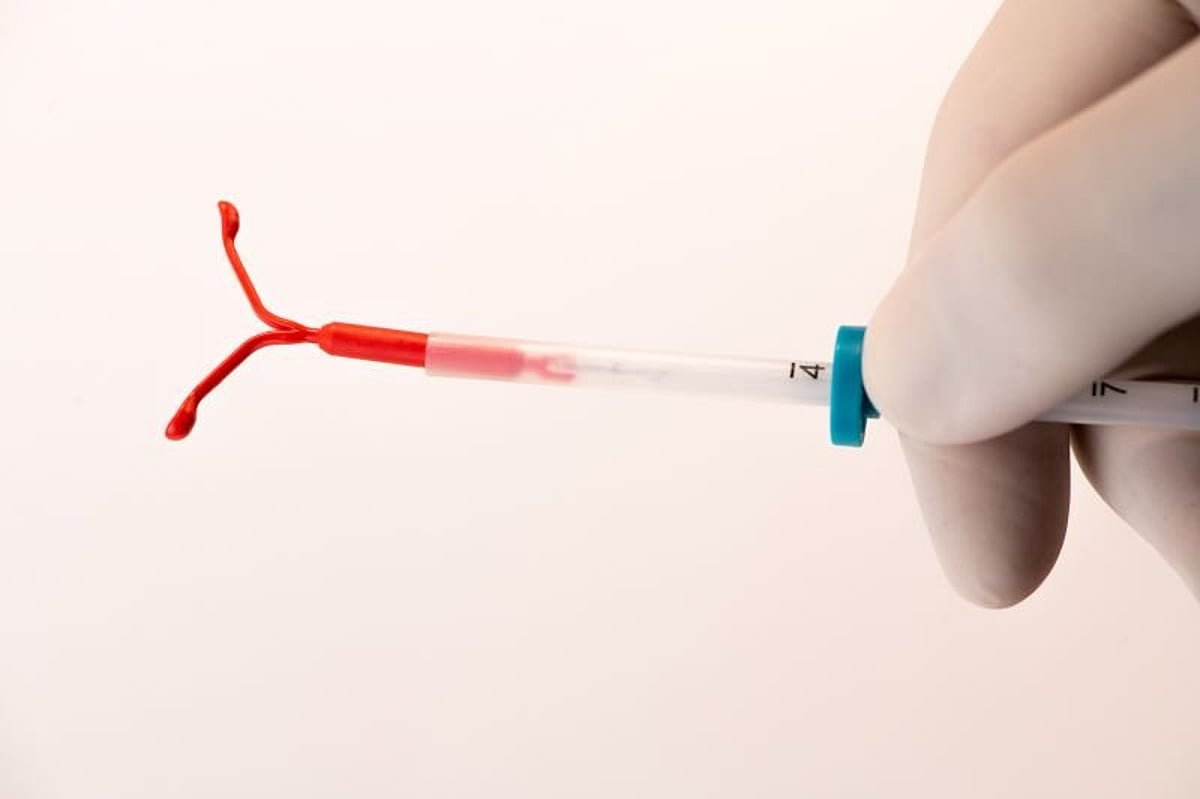
THURSDAY, March 23, 2023 (HealthDay News) -- Intrauterine device (IUD) placement at two to four weeks postpartum is noninferior to placement at six to eight weeks postpartum for complete expulsion, according to a study published in the March 21 issue of the Journal of the American Medical Association.
Sarah Averbach, M.D., from the University of California San Diego in La Jolla, and colleagues conducted a randomized, noninferiority trial involving people who had a vaginal or cesarean birth to assess expulsion rates for IUDs placed early postpartum compared to those placed at the standard interval six-week visit. A total of 404 participants were enrolled and randomly assigned to early IUD placement (14 to 28 days; 203 participants) or interval IUD placement (42 to 56 days; 201 participants).
The primary outcome was complete IUD expulsion by six months postpartum; the noninferiority margin was 6 percent. Of the 294 participants who received an IUD and completed six-month follow-up, the researchers found that the complete expulsion rates were 2.0 and 0 percent in the early and interval placement groups, respectively (between-group difference, 2.0 [95 percent confidence interval, −0.05 to 5.7] percentage points). Partial expulsion occurred in 9.4 and 7.6 percent of participants in the early and interval placement groups, respectively (between-group difference, 1.8 [95 percent confidence interval, −4.8 to 8.6] percentage points). At six months, IUD use was similar between the groups (69.5 and 67.2 percent, respectively).
"The early placement group in this study had a small absolute increase in risk of partial expulsion, which did not meet the prespecified criterion for noninferiority," the authors write.
One author disclosed financial ties to Bayer Pharmaceuticals.
Abstract/Full Text (subscription or payment may be required)
Related Posts
Dental Phobia
Tim Day is a master sergeant in the Air Force. When the Gulf War erupted in the...
Heart Disease When Young Could Bring Memory Issues by Middle Age
THURSDAY, Jan. 26, 2023 (HealthDay News) -- People who suffer a heart attack or...
AHA News: What Parents Should Know About the COVID-19 Vaccine For 5- to 11-Year-Olds
FRIDAY, Nov. 5, 2021 (American Heart Association News) -- A COVID-19 vaccine has...
