FDA Authorizes Pfizer Booster Shot for Children Ages 5 to 11 Years
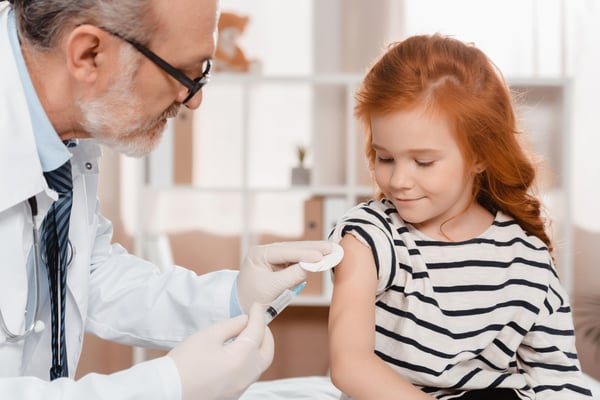
TUESDAY, May 17, 2022 (HealthDay News) -- A single booster dose of the Pfizer COVID-19 vaccine can be given to 5- to 11-year-olds, the U.S. Food and Drug Administration announced today.
The third shot can be given at least five months after healthy children complete the two-dose vaccine series, the FDA said. Its action, which now goes to the U.S. Centers for Disease Control and Prevention for approval, dovetails with a rise in infections in many areas of the country.
"While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease," FDA Commissioner Robert Califf, M.D., said in a statement. "The FDA is authorizing the use of a single booster dose of the Pfizer-BioNTech COVID-19 vaccine for children 5 through 11 years of age to provide continued protection against COVID-19."
FDA officials based their decision on an analysis of data from a group of children in an ongoing trial that led to last fall's authorization of the Pfizer vaccine primary series in 5- to 11-year-olds. Antibody responses were evaluated in 67 study participants who received a booster dose seven to nine months after completing the two-dose vaccine regimen. A month after the booster, their antibody levels were higher than before, according to the FDA.
The safety of a single Pfizer booster dose was assessed in about 400 children, aged 5 to 11 years, who received it five to nine months after the two-dose series. The most commonly reported side effects were pain, redness and swelling at the injection site, as well as fatigue, headache, muscle or joint pain, and chills and fever.
Califf urged parents whose eligible children have not yet been vaccinated to get the shots. "Getting them vaccinated can help protect them from the potentially severe consequences that can occur, such as hospitalization and death," he said in the FDA news release.
Related Posts
Experiment Shows Many Seniors Falling Prey to ‘Impostor Scams’
MONDAY, Sept. 25, 2023 (HealthDay News) -- Many older adults are savvy about...
The Most Common Depression Medications, Explained
FRIDAY, May 26, 2023 (HealthDay News) -- You've been diagnosed with depression....
Review Looks at Psychological Burden of Children, Young Adult Cancer Patients
THURSDAY, June 29, 2023 (HealthDay News) -- Children, adolescent, and young...
FDA Withdraws Approval of Drug Meant to Prevent Preterm Births
THURSDAY, April 6, 2023 (HealthDay News) -- The U.S. Food and Drug...
